原标题:2015年12个医用塑料的大事儿,有哪些是你不知道的?
又到一年最后一个月月中了,这一年塑料领域发生了很多事儿,小编在这里想说,塑料是奇妙的,在2015年在医疗领域尤其如此。从世界各地的研究机构到材料供应商和医疗设备OEM制造商,进步和突破不断进行着。
在此,小编特别给大家整理了2015年非常特别的医用塑料。
1、美国第一位病人接受塑料半月板移植今年1月,2015年1月,外科医生(俄勒冈州立大学)俄亥俄州立大学医学中心(哥伦布,,OH)实施由美国fda批准的第一个塑料半月板植入临床试验。该装置非常有能力给半月板撕裂患者提供微创疗法,进而克服了许多目前治疗的局限性。
@ In January 2015, surgeons at Ohio State University (OSU) Medical Center (Columbus, OH) performed the first plastic meniscus implantation in the United States as part of an FDA-approved clinical trial. The device has the potential to offer patients with a torn meniscus a minimally invasive remedy that overcomes many of the limitations of current treatments
2、突破性生物材料技术,为可吸收植入代开新途径Proxy Biomedical (爱尔兰高威) 研发了生物材料加工技术开发,并声明其提高了材料强度,目前用于生产可吸收的骨科植入,应用率达100%以上。通过增加可吸收性材料的强度和韧性, Proxy Bio-XT可以说是一种为植入设计的颠覆性技术。
@A proprietary biomaterial processing technology developed by Proxy Biomedical (Galway, Ireland) reportedly boosts the strength of materials currently used to manufacture resorbable orthopedic implants by more than 100%.
3、注射型塑料可以在偏远地区作为救生使用由华盛顿大学的研究人员开发(西雅图华盛顿大学;) 的一种新的注射聚合物,可以加强血液凝块, 在战场上挽救无数的生命,还有适用于农村地区和其他医疗可能不可直接到达的地方。PolySTAT就像factor XIII, 它是身体中的一种天然蛋白质,帮助加强血液凝块,通过交联纤维蛋白链形成伤口,防止出血。研究人员报告,材料可能会比当前使用的需要仔细存储的blood-based产品便宜.(详细内容见:美国发明注射止血聚合物材料PolySTAT)
@A new injectable polymer developed by researchers at the University of Washington (UW; Seattle) could strengthen blood clots and save countless lives on the battlefield, in rural areas and anywhere else where immediate medical treatment may not be available.
4、纤维塑料改善增强设备通常应用于汽车和航空航天, 根据生产技术IPT的弗劳恩霍夫研究所(德国亚琛), 纤维增强塑料在医学应用方面也有巨大的潜力。研究所发现一种微粒曲折过程,可以使微创设备大规模生产。
@Typically used in automotive and aerospace applications, fiber-reinforced plastics also have enormous potential in medical applications, according to the Fraunhofer-Institute for Production Technology IPT (Aachen, Germany).
5、FDA批准首个3D打印聚合物移植,应用于承载领域牛津性能材料有限公司为SpineFab系统从FDA收到510(k)空隙,用于长期植入的承载聚合物装置。旨在取代碰撞造成损坏或不稳定的椎体肿瘤或创伤,VBR系统是第一个也是唯一一个FDA通过的3 d打印设备。
@Oxford Performance Materials Inc. (South Windsor, CT) has received 510(k) clearance from FDA for its SpineFab system, a load-bearing polymer device for long-term implantation.
6、导电聚合物涂层提高设备性能医疗设备涂层技术,旨在加强人体组织和由Biotectix引入的电极之间界面的沟通。Amplicoat技术声明其克服了其他导电涂料在医学应用的局限性,并改进其耐用性,加工性和性能。
@Medical device coating technology that is designed to enhance communication at the interface between human tissue and electrodes was introduced by Biotectix (Ann Arbor, MI) at the start of the year.
7、FDA批准首个生物可吸收性聚合物药物洗脱支架波士顿科学(马尔伯勒,MA)周一宣布,已收到美国食品及药物管理局批准其协同生物吸收性聚合物药物洗脱支架系统(BP-DES),用于治疗冠状动脉疾病。这是第一个也是唯一一个BP-DES批准使用在美国。进入国内支架市场,其每年市值13亿美元,在全球范围内,市场价值40亿美元.
@Boston Scientific (Marlborough, MA) announced on Monday that it has received FDA approval for its Synergy Bioabsorbable Polymer Drug-Eluting Stent System (BP-DES) for the treatment of coronary artery disease. It is the first and only BP-DES approved for use in the United States, entering a domestic stent market that is valued at $1.3 billion annually; globally, the market is worth $4 billion.
8、摩擦测试数据,减少原材料采购的工作Compounder RTP Co. (威诺娜,MN)在几年年初公布摩擦数据,针对于医疗设备的设计者与MD&M West参与者使用新的摩擦试验方法、RTP工程师能够评估基础树脂在各种组合的减阻添加剂。收集到的数据从这些测试可以消除试错测试时对一次性医疗器械采购聚合物。
@Friction test data eliminates material-sourcing guesswork
(Winona, MN) unveiled the results of tribology data for designers of medical devices to attendees of in Anaheim, CA, and NPE2015 in Orlando, FL earlier this year.
9、聚乳酸和聚乙醇酸造成的secant 医学中可吸收技术的限制聚乳酸(PLA)和聚乙醇酸(PGA)是最常见的首选材料用于制造的可吸收性植入物。然而,他们有缺点:PLA和PGA可能引起人体炎症,因为他们是硬的材料,他们不符合人体组织的弹性性能。Secant Medical的基于聚癸二酸丙三醇酯(PGS)新生技术提供了一个选择
@Bioresorbable technology from Secant Medical addresses limitations of PLA and PGA Poly lactic acid (PLA) and poly glycolic acid (PGA) are the most common go-to materials for the fabrication of resorbable implants. They have drawbacks, however: PLA and PGA may cause inflammation and, because they are stiff materials, they do not conform well to the elastic nature of tissue.
10、硬性PVC 等同于聚碳酸酯的性能Teknor Apex (Pawtucket, RI)开发的刚性PVC可以取代聚碳酸酯(PC),在医疗组件方面如连接器和止回阀等, 在减少或消除应力开裂时,明显与PC比较该材料达到强度。(详细内容见这里:硬质PVC取代PC在医用连接器中的应用)
@Rigid PVC equals performance of PC
A rigid PVC developed by Teknor Apex (Pawtucket, RI) can replace polycarbonate (PC) in clear medical components such as connectors and check valves, according to the company. The material achieves strength and clarity comparable to PC while reducing or eliminating the stress cracking that often occurs in PC at the interface with flexible PVC tubing and other components.
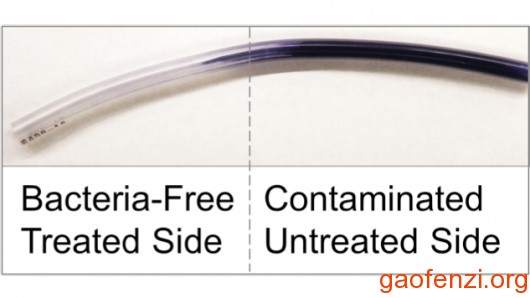
11、液体注入型聚合物,防止医疗设备上生物膜的形成生物工程研究所的研究人员(波士顿)2015年2月宣布,他们已经开发了一种聚合物,该聚合物分子结构中储存大量的润滑液体和随着时间释放他们,呈而使材料不断光滑并排斥细菌。他们做了一个实验,用通常用于医疗的固体硅胶聚合物通过油管注入硅油。“固体硅胶管在硅油中饱和, 在其分子结构浸泡成所有的小空间,这两种材料真正集聚成一种,”研究员凯特琳豪厄尔说。饱和过程中产生液体注入型聚合物,这个方法足够作为常规灭菌方法。(详细内容见:【文献】美发明新型抗菌导管技术)
@Liquid-infused polymers prevent biofilm formation on medical devices
Researchers at the Wyss Institute for Biologically Inspired Engineering (Boston) announced in February 2015 that they had developed polymers that store considerable amounts of lubricating liquids within their molecular structure and release them over time to render the material continuously slippery and repellant to bacteria. They conducted an experiment using a solid silicone polymer that is typically used in medical tubing infused with a silicone oil.
12、Covestro聚碳酸酯特殊处理,成专用可穿戴式医疗设备虽然在可穿戴传感器只让人关注医疗器械技术, 但材料也起着重要的作用, 因为消费者不会穿刺激皮肤或只是舒服的东西哦。今年早些时候,拜耳材料科学(德国勒沃库森,),现在被称为Covestro,介绍了聚碳酸酯满足混合的独特需求,用于可穿戴的生命体征监测,药物传输设备和相关产品。
@Covestro debuts PC blend formulated specifically for wearable medical devices
Although sensors seem to get all the attention in wearable medical device technology, materials also play an important role, since consumers probably won’t wear something that irritates their skin or is just plain uncomfortable. Earlier this year, Bayer MaterialScience (Leverkusen, Germany), now known as Covestro, introduced a polycarbonate blend that satisfies the unique requirements of wearable vital signs monitors, drug-delivery devices and related products.
本文来源公众号:AsiaSolution,仅供参考学习
(部分内容和图片小编增加)